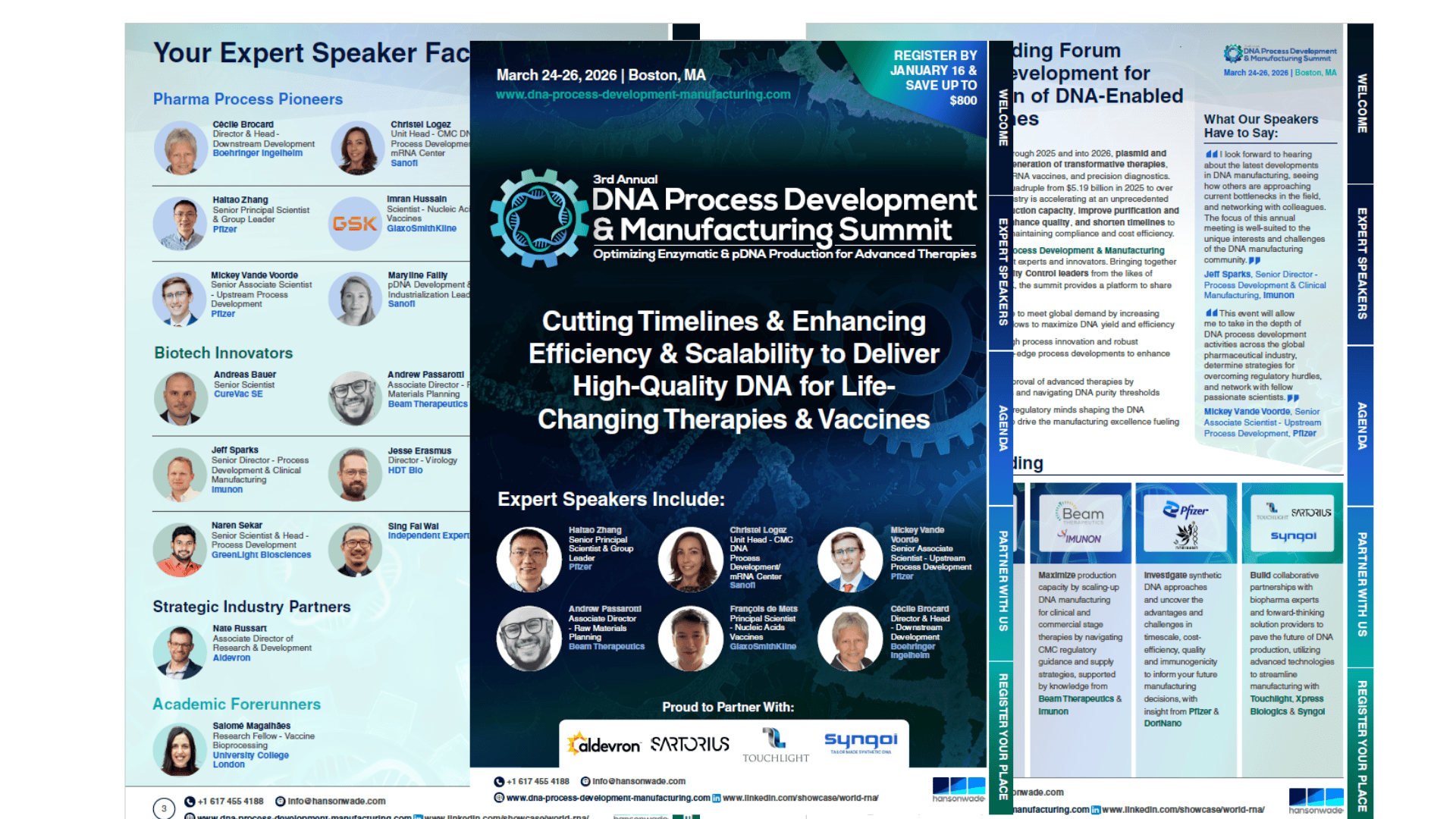
Cutting Timelines & Enhancing Efficiency & Scalability to Deliver High-Quality DNA for Life Changing Therapies and Vaccines
Cutting-edge breakthroughs in DNA production, from high-yield plasmid manufacturing to next-generation cell-free platforms, are transforming how biopharma develops advanced vaccines and nucleic acid–based therapeutics. Organizations across the space are accelerating investment in technologies that enhance upstream fermentation efficiency, streamline downstream purification, and strengthen CMC frameworks for rapid, compliant scale-up.
The 3rd DNA Process Development & Manufacturing Summit arrives at this pivotal moment as the only meeting entirely focused on DNA process development and manufacturing, designed to immerse you in the applied learnings needed to drive the next wave of innovation in DNA production.
Delve into the latest approaches for optimizing plasmid design, boosting fermentation productivity, advancing purification, reducing impurities, building robust analytical and QC strategies, and aligning regulatory expectations from early development through commercial production. Explore how to unlock reproducibility, scalability, and cost-efficient GMP-grade DNA.
Unite with industry leaders such as Sanofi, Pfizer, GSK, Boehringer Ingelheim, GreenLight Biosciences, HDT Bio, and others, as well as innovative emerging players advancing new DNA formats and manufacturing technologies.
This is your premier meeting dedicated to exploring the full DNA manufacturing lifecycle, from upstream to downstream, process development to scale-up, and quality to regulatory alignment. Walk away with actionable insights, practical solutions, and new strategic partnerships to accelerate the delivery of high-quality DNA for tomorrow’s vaccines and therapies.
Attending Companies Include